Psychedelics Promote Neural Plasticity
A new study from the University of California, Davis has found that psychedelic drugs such as LSD and DMT promote neural plasticity and development, indicating a potential mechanism for their therapeutic benefits.
Patients who suffer from depression and post-traumatic-stress-disorder tend to have impaired neurogenesis and neuroplasticity – their brain cells grow more slowly and are less adaptable. These structural changes can lead to atrophy of various brain regions, including the hippocampus (which is involved in learning and memory) and the prefrontal cortex (which mediates personality and decision-making).
Counteracting this damage by promoting structural and functional neural plasticity has been suggested as novel way of treating psychiatric disorders. However, relatively few compounds that promote neuroplasticity – which the authors of the new study term ‘psychoplastogens’ – have been found capable of achieving this without drawbacks.
Ketamine, a dissociative anaesthetic with hallucinogenic properties, is a notable exception. By activating pathways involved with forming neurone connections, it has been found to be an extremely effective therapeutic for treatment-resistant depression (figure 1b).
Similarly, the Beckley/Imperial Psychedelic Research Programme have demonstrated significant and even longer-lasting benefits of psilocybin in the treatment of depression (figure 1a).
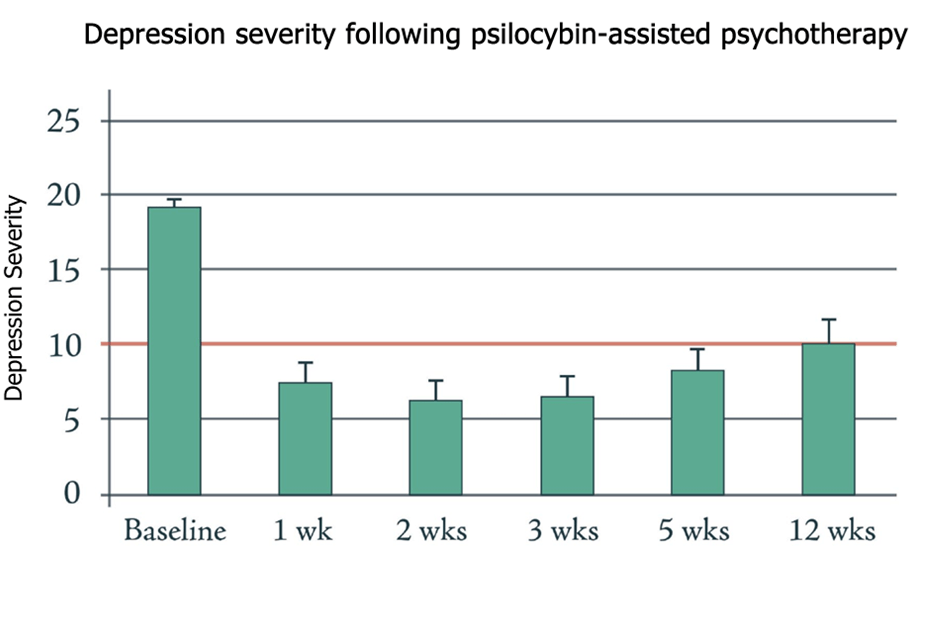
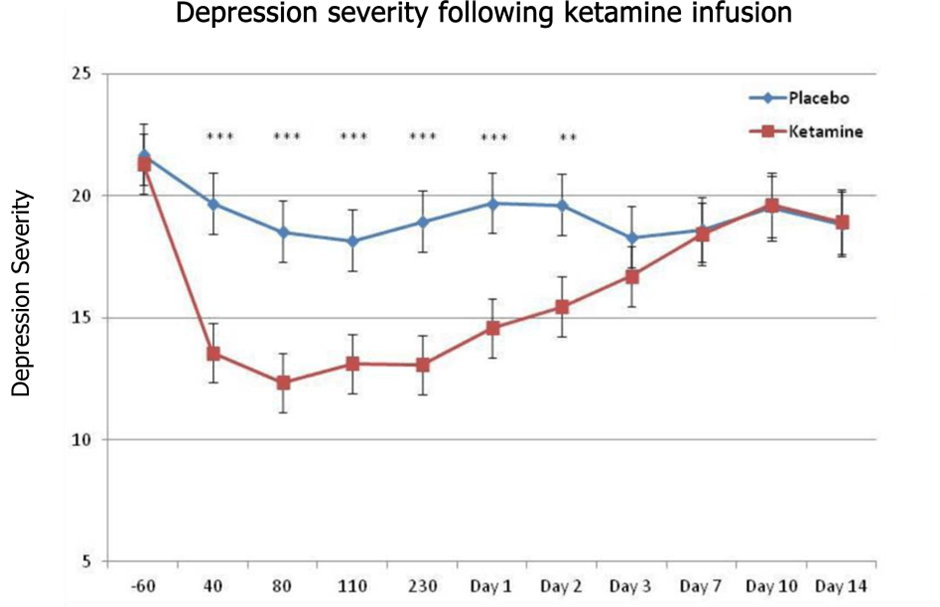
Fig.1a – Psychedelics have a significant, long-lasting benefit in treating depression (adapted from Carhart-Harris et al. 2016). Fig.1b – Ketamine has similarly shown promise in treatment-resistant depression, though effects do not last as long as those observed with psilocybin (from Zarate et al. 2012).
This new study hoped to elucidate the cellular mechanism by which psychedelics achieve their therapeutic effect by investigating whether and how they affect neural growth and plasticity.
In the first experiment, scientists treated cultures of cortical neurones with psychedelics, and then observed how the neurones developed and increased in complexity (figure 2).
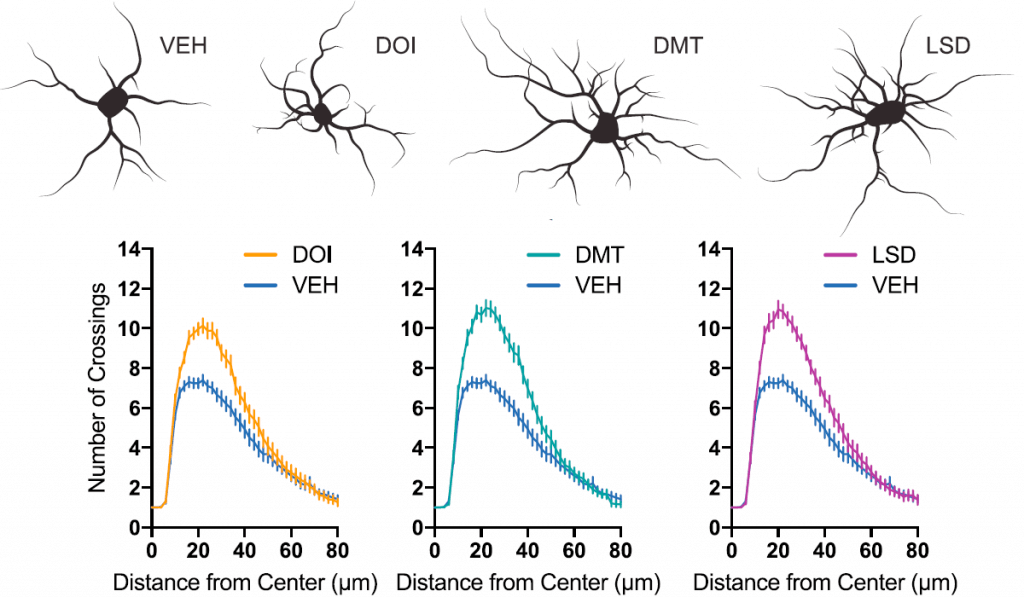
Fig.2 – Psychedelics significantly increased complexity of cortical neurones compared to VEH – no drug. This was measured by analysing how often cell branches and offshoots (neurites) crossed over each other at various distances from the cell centre. The figure includes a representative tracing of a cell from each treatment (Ly et al. 2018)
They found that LSD, DMT, and DOI – all serotonergic psychedelics – significantly increased the growth and complexity of neurones in a similar manner to ketamine, with LSD particularly potent. Interestingly, ibogaine was found to have no effect on neuroplasticity – but its metabolite noribogaine did, suggesting it was the active molecule in the anti-addictive properties of iboga.
As a comparison, amphetamine and serotonin – which share structural similarities with psychedelics – were also tested, and were found to have no effect on measures of neurogenesis.
These effects were observed not only in cell cultures, but also by testing the compounds on the brains of fly larvae and zebrafish, showing that they also have a tangible effect in living organisms.
In a separate measure of neural plasticity, psychedelics were found to significantly increase the number of dendritic spines on cortical neurones, with LSD almost doubling their density (figure 3). These spines form synapses with other neurones and are a major site of molecular activity in the brain. Their functioning is closely related to higher cognition, and loss of these structures is a hallmark of depression and other neuropsychiatric disorders.
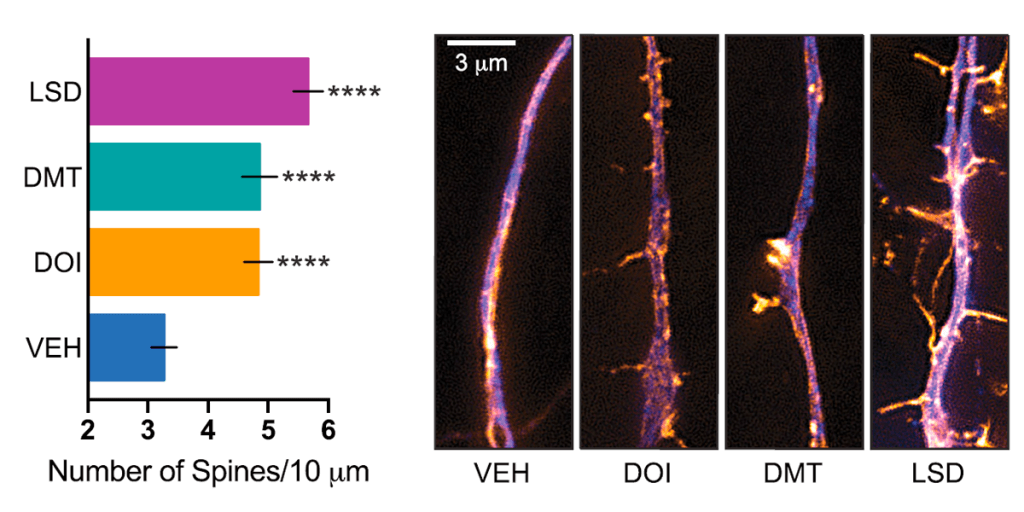
Fig.3 – Effect of psychedelics on spinogenesis and synaptogenesis. The number of dendritic spines on cortical neurones – which act as gateways and connections to other neurones – was significantly increased following treatment with psychedelics (Ly et al. 2018)
The positive effects were not only structural, but functional – electrophysiological recordings found that the frequency and strength of neural currents were increased for many hours after the psychedelic compounds had been removed.
The study then further elucidated the molecular mechanisms involved. mTOR is a key component of internal neural signalling pathways, involved with recruiting receptors and forming synapses, and is also known to be the target through which ketamine achieves its antidepressant effect. When mTOR was blocked, the psychoplastogenic effects of psychedelics seen above were also inhibited, indicating that they achieve their effect through a similar mechanism to ketamine.
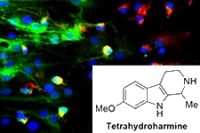
This study builds on previous findings by the Beckley/Sant Pau Research Programme, which observed that components of the psychedelic brew ayahuasca promoted growth and maturation of neurones (figure 4). Ayahuasca has also been demonstrated to have significant anti-depressant effects, further suggesting neuroplasticity as a common mechanism for the actions of ketamine and psychedelics like LSD and ayahuasca. The Beckley Foundation is determined to investigate this further, both in vitro and in humans, as part of our Research Programmes with Brazil, Maastricht University and Imperial College London.
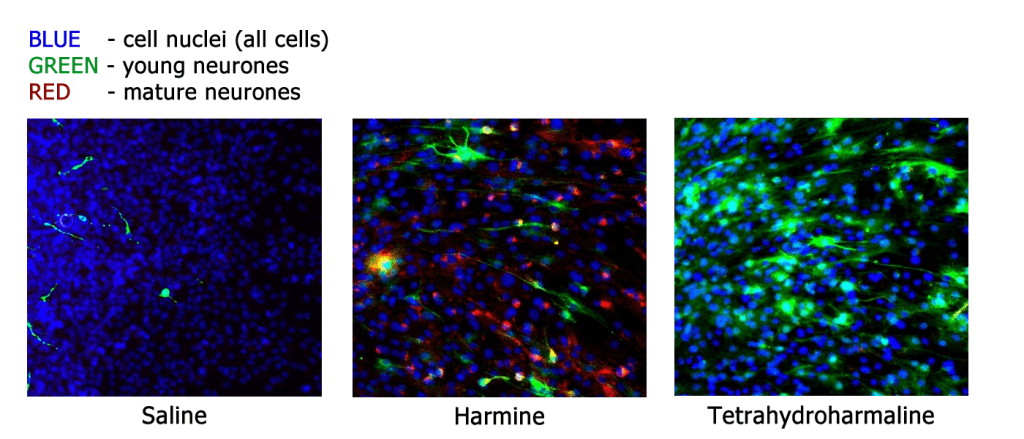
Fig.4 – Components of the psychedelic brew ayahuasca promote growth and maturation of neurones, demonstrated by cell-cycle-specific staining of neurones in cell culture (from Morales-Garcia et al. 2017).
These findings add yet more scientific evidence for the therapeutic benefit of psychedelic compounds. Not only have psychedelics been demonstrated to have ground-breaking potential in treating previously untreatable psychiatric disorders, but they are almost unique in their ability to promote neuroplasticity in a safe way, healing both mind and brain.
Sadly, psilocybin, LSD, DMT, and other psychedelics remain on Schedule I of both the UK and the UN’s global drug conventions, severely limiting both research and clinical application. The Beckley Foundation is actively campaigning to have psychedelics and other psychoactive medicines re-scheduled, so that doctors and psychotherapists may use them as a tool to help heal those who are not benefiting from currently available treatments. Despite restrictions, we continue to collaborate with research groups all over the world to better understand these compounds – together, we hope to create a paradigm shift in psychiatry which will benefit all of society.
Nick Cherbanich
Podcast
- All
Links
- All
Support
- All
BIPRP
- All
Science Talk
- All
Amanda's Talks
- All
- Video Talk
- Featured
- 2016 Onwards
- 2011-2015
- 2010 and Earlier
- Science Talk
- Policy Talk
One-pager
- All
Music
- All
Amanda Feilding
- All
Events
- All
Highlights
- All
Psilocybin for Depression
- All
Current
- All
Category
- All
- Science
- Policy
- Culture
Substance/Method
- All
- Opiates
- Novel Psychoactive Substances
- Meditation
- Trepanation
- LSD
- Psilocybin
- Cannabis/cannabinoids
- Ayahuasca/DMT
- Coca/Cocaine
- MDMA
Collaboration
- All
- Beckley/Brazil Research Programme
- Beckley/Maastricht Research Programme
- Exeter University
- ICEERS
- Beckley/Sant Pau Research Programme
- University College London
- New York University
- Cardiff University
- Madrid Computense University
- Ethnobotanicals Research Programme
- Freiburg University
- Medical Office for Psychiatry and Psychotherapy, Solothurn
- Beckley/Sechenov Institute Research programme
- Hannover Medical School
- Beckley/Imperial Research Programme
- King's College London
- Johns Hopkins University
Clinical Application
- All
- Depression
- Addictions
- Anxiety
- Psychosis
- PTSD
- Cancer
- Cluster Headaches
Policy Focus
- All
- Policy Reports
- Advisory Work
- Seminar Series
- Advocacy/Campaigns
Type of publication
- All
- Original research
- Report
- Review
- Opinion/Correspondence
- Book
- Book chapter
- Conference abstract
- Petition/campaign
Search type